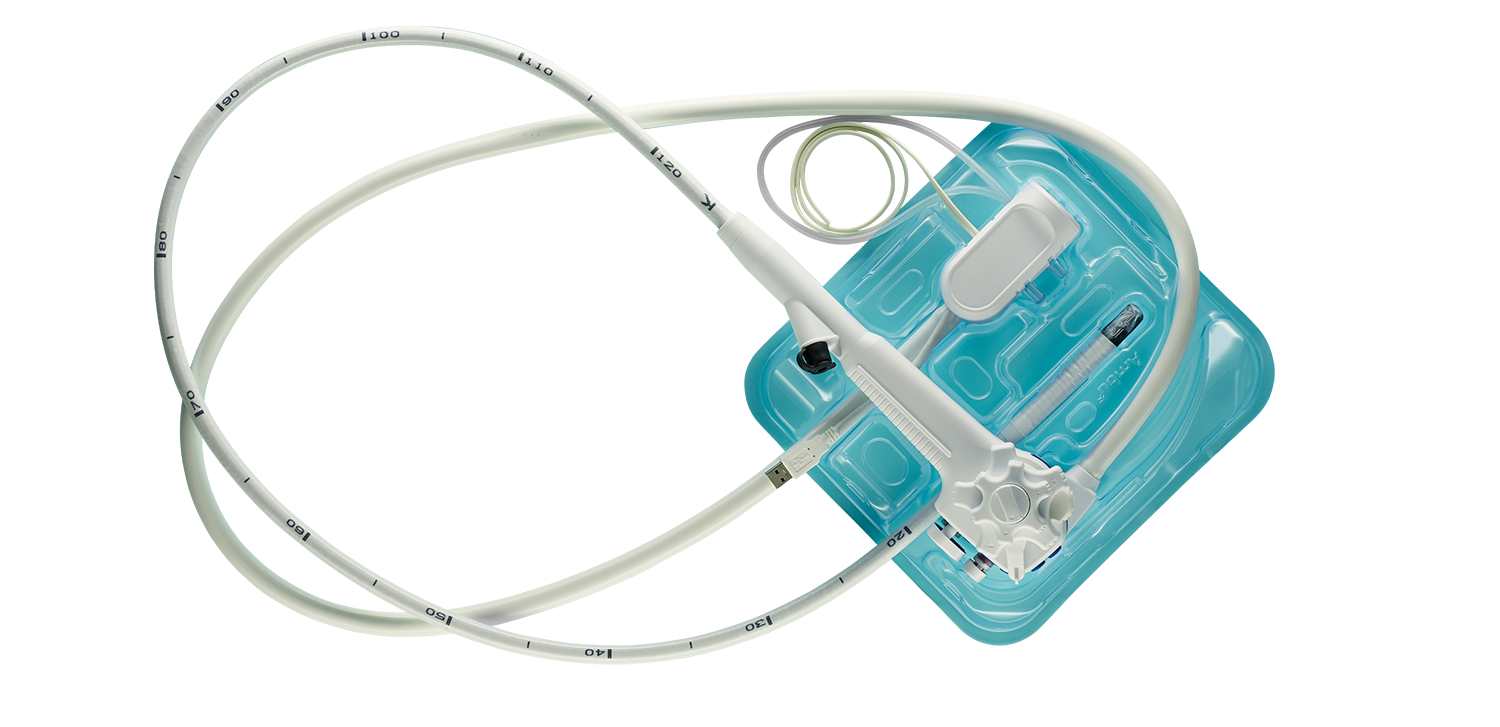
Eliminate reprocessing, repairs, patient cross contamination, and reduce cost/capital

Even with the most advanced reprocessing, there is always a risk, however small, of patient cross-contamination. The FDA has called for an innovative solution to this issue. aScope Duodeno solves the problem.
aScope Duodeno is a fully disposable, single-use duodenoscope for ERCP procedures. It helps you address concerns about patient cross-contamination and patient safety.
Sterile straight from the pack
aScope Duodeno eliminates the risk of patient cross-contamination because you receive a new sterile scope straight from the pack for every procedure.
No need for reprocessing or repair
With single-use, there is no need for reprocessing or repair. As a result, you can free up staff to focus on other valuable tasks.
A seamless transition
The single-use aScope Duodeno is designed to deliver the performance of conventional duodenoscopes.
Breakthrough Device Designation
In July, 2020, the aScope Duodeno endoscope received FDA clearance and was also granted Breakthrough Device Designation. The FDA's Breakthrough Device Program is reserved for certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. This program is intended to help patients receive more timely access to these medical devices by expediting their development, assessment and review by FDA.
The True Cost of Reusable Duodenoscopes
Reusable duodenoscopes are $40,000 and used in patients for 5+ years at a time before being replaced. The aScope Duodeno is used just once. Watch the video above to compare the costs of reusable versus single-use duodenoscopes.
KEY BENEFITS
Ambu® aScope™ Duodeno
Spare parts
There are no spare parts or accessories for this product.Downloads
Brochures
(4.23 MB - pdf)
(3.06 MB - pdf)
(5.12 MB - pdf)
Datasheets
(549.81 KB - pdf)
In-Service Videos
(14.48 MB - mp4)
(8.76 MB - mp4)
(20.25 MB - mp4)
Instructions for use
(26.12 MB - pdf)
Supplementary information (5)
(2.06 MB - pdf)
(693.6 KB - pdf)
(2.56 MB - pdf)
(1.51 MB - pdf)
(4.32 MB - pdf)
May 2020
Note: US: Rx only